Research
Fat Talks
Learn from ‘Pat’ the pre-fat cell, what the Hilgendorf Lab studies.
Research Overview
Signals
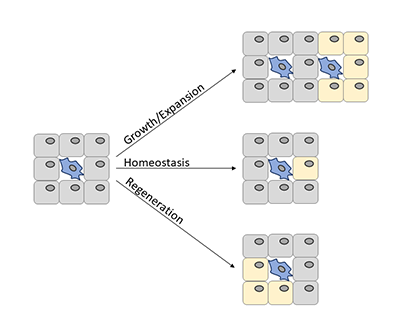
Tissue maintenance requires stem and progenitor cells to respond to a wide range of signals. We want to understand on a molecular level what these signals are, how they inform cell fate, and how alterations drive pathogenesis. To do this, we use cell and molecular biology techniques, biochemistry, mouse genetics, and multi-omic technologies.
The Primary Cilium
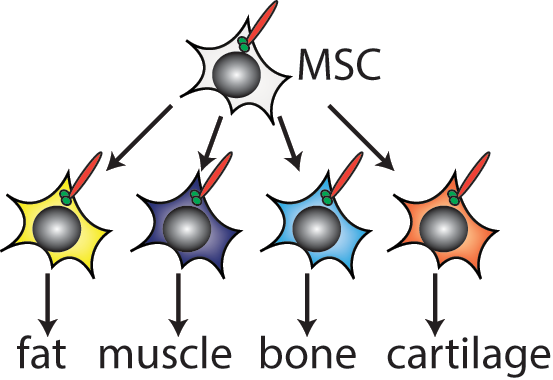
One major focus in the lab is the primary cilium, a central sensory organelle of the cell. Mesenchymal stem and progenitor cells, which give rise to fat, muscle, bone, and cartilage, are all ciliated. We and others have shown that loss of ciliation on mesenchymal progenitor cells impairs tissue maintenance, repair, and expansion. Human genetic mutations leading to dysfunctional cilia (ciliopathies) can manifest obesity, diabetes, hypotonia, and skeletal dysplasia.
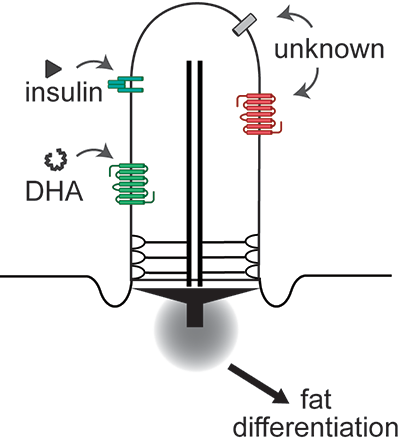
We want to understand how primary cilia on stem and progenitor cells regulate differentiation, including which physiological signals they sense, how these get communicated to the transcriptional machinery of the cell, and how dysfunctional ciliary signaling contributes to pathogenesis. By focusing on signaling pathways that are mediated by the primary cilium, we can (a) leverage advances in human genetics associated with ciliopathies to drive new hypotheses, (b) exploit the specific localization of ciliary receptors to mechanistically interrogate these signaling pathways, and (c) begin to understand how the primary cilium integrates signals for binary cell fate decisions.
Fat Differentiation and Metabolic Health
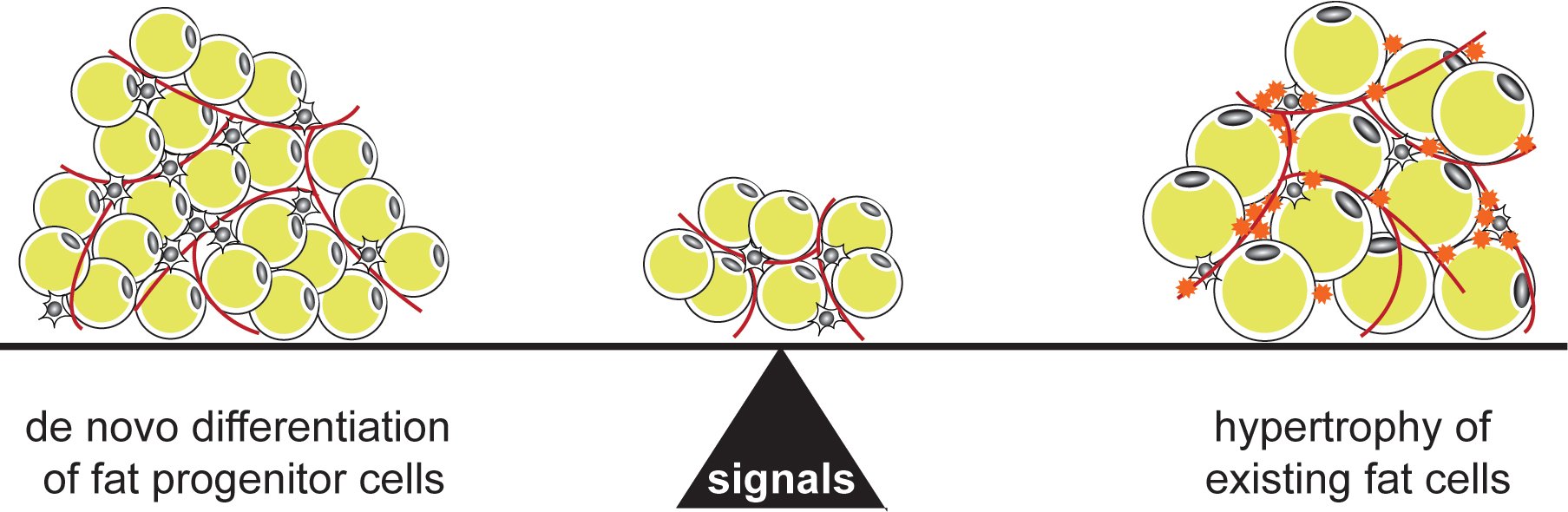
We have a strong interest in studying fat differentiation and white adipose tissue expansion. The prevalence of obesity, defined as excess white adipose tissue, has reached pandemic levels and its comorbidities, including diabetes and cancer, pose a significant challenge to public health. Adipose tissue expands by generating more fat cells as well as storing more lipids in existing fat cells. The relative balance between these two mechanisms has a profound effect on metabolic health: Larger fat cells are associated with insulin resistance and inflammation leading to diabetes, while white adipose tissue containing smaller fat cells, even if greater in number, is considered metabolically healthy. We want to identify the physiological and endocrine signals that regulate how white adipose tissue expands, and how we can shift the balance to metabolically healthy white adipose tissue expansion.
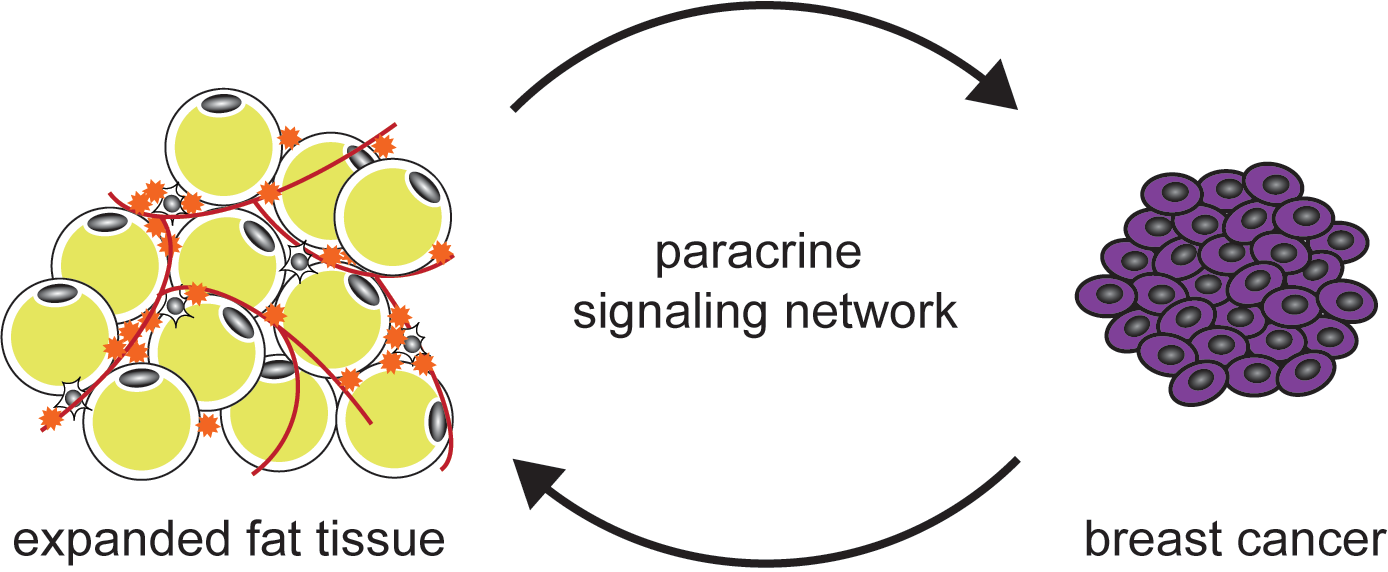
Obese Fat Tissue and Breast Cancer
Obesity is a known risk factor for 13 types of cancer, including post-menopausal breast cancer. We are studying the localized, reciprocal interaction between breast cancer cells and cells in the mammary fat tissue. We want to identify the paracrine signals secreted by breast cancer cells to remodel its microenvironment, and the paracrine signals secreted by the mammary fat tissue to support breast cancer growth.